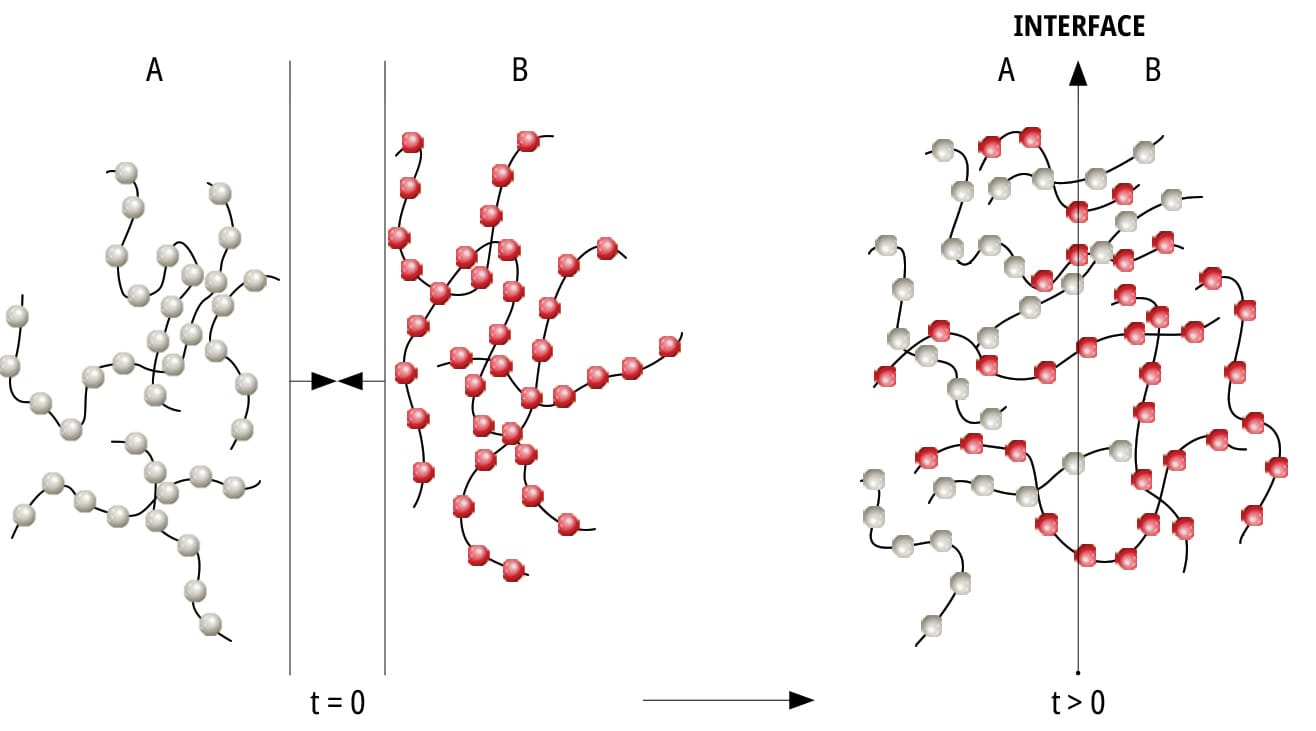
4.1.2 Diffusion Theory
Diffusion theory is applicable for long-chain polymers that are capable of movement. Adhesion is the result of the diffusion of molecules between the adhesive and the surface. Depending on the adhesive’s chemical properties, operational conditions and substrate, the diffusion will vary in depth.
4.1.3 Wetting and Polarity
Surface energy is the degree of attraction or repulsion exerted by the surface of one material to another (measured in mN/m or outdated dyn/cm). Surface tension is a liquid’s resistance to surface penetration, which allows the liquid to behave like a thin elastic film. The wetting theory states that adhesion results from the surface forces of molecular contact. In the ideal bonding condition, a substrate has high surface energy, i.e. the surface has the capacity to attract, and the adhesive has a low surface tension, i.e. low resistance to deformation or breakage. This results in the adhesive properly wetting the substrate and forming a strong bond.12 Surface preparation for bonding frequently increases the surface energy of a substrate to improve bond strength.
12) S. Ebnesajjad, Handbook of adhesives and surface preparation, Technology, Applications and Manufacturing (2011).
| SUBSTRATE | FREE ENERGY (mN/m) | SUBSTRATE | FREE ENERGY (mN/m) | SUBSTRATE | FREE ENERGY (mN/m) |
| Iron | 2,550 | Tin | 700 | Polyvinyl Chloride | 40 |
| Nickel | 2,450 | Glass | 300 | Polycarbonate | 35 |
| Titanium | 2,050 | Water | 73 | Polystyrene | 33 |
| Copper | 1,850 | Polyimide | 50 | Polyethylene | 31 |
| Gold | 1,550 | Polyamide | 49 | Polypropylene | 29 |
| Silver | 1,250 | Epoxy Resin | 47 | Natural Rubber | 24 |
| Aluminium | 1,200 | Polyamide 6.6 | 46 | Silicone | 24 |
| Zinc | 1,020 | Polyethyleneterephtalate | 43 | Polytetrafluoroethylene | 18 |
The surface polarity of the surface is directly related to the wetting and surface energy and affects the polymerisation reaction rate and the joint strength on a substrate. If the surface is polar, it is very reactive and will become stable after the reaction. Surface polarity is strongly related to surface free energy. Surfaces with high free energy have high polarity.
Testing Surface Energy
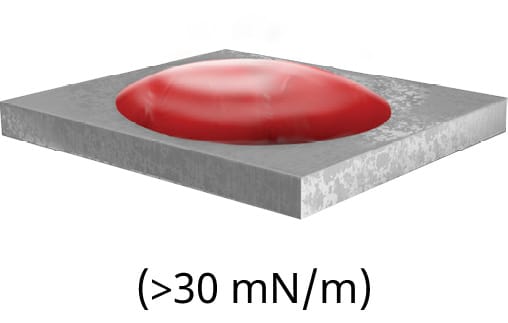
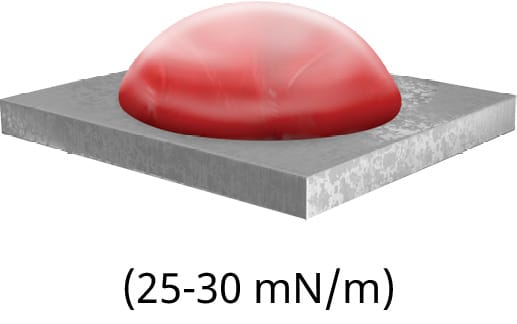
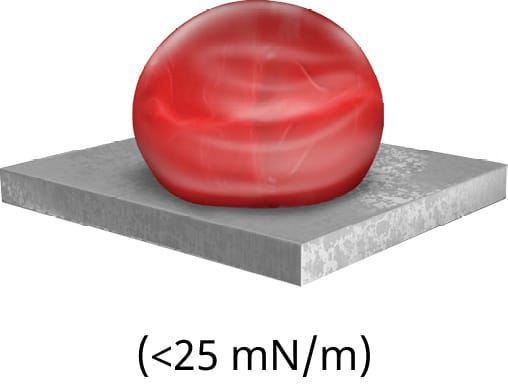
If the particular material does not have a stated surface energy, a quick and easy ‘ink test’ can be conducted to approximate the surface energy. The ink test is a series of solutions containing a variety of surfactants that are calibrated to specific surface tension value ranges. Each ink should be applied in succession until the appropriate level of dispersion is reached. However, this test is simply an approximation of the surface energy. To measure the exact surface energy of a substrate, the contact angle should be measured, as shown in Figure 79.
A) displays poor wetting with a large contact angle
B) shows good wetting with a lesser contact angle
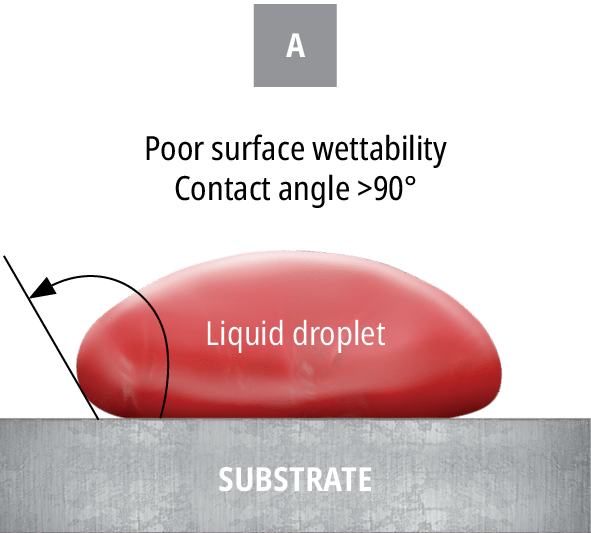
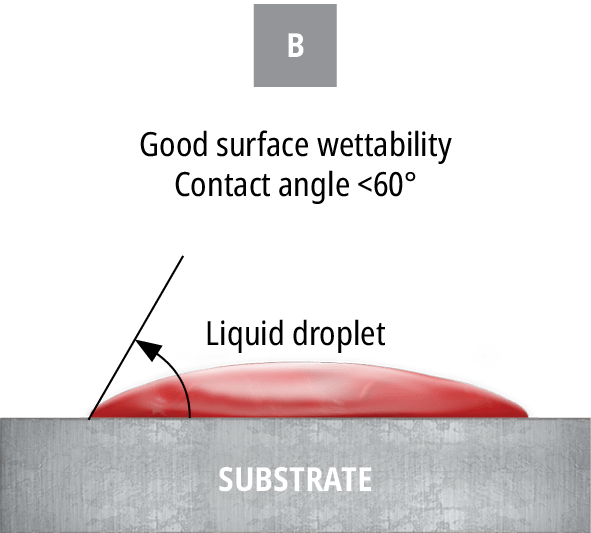
Put simply, surface energy is the ability of a solid surface to wet with a liquid, while surface tension is the ability of an adhesive to wet a solid surface. Based on the difference in a surface’s energy and the surface tension of the adhesive, the attraction between the liquid and the solid can be measured by observing the contact angle.
The smaller the contact angle, the more attracted a liquid is to a surface and the likelier it is to wet out. An example of this in everyday life is the beading of water droplets that occurs after a fresh wax job on the exterior of a car. The wax layer effectively decreases the surface energy, leading to a decrease in attraction between the water droplets and the paint. This phenomenon can be explained by Van Der Waals forces, which are intermolecular attractive forces.13 Not only will the surface energy of a material affect the ability of the adhesive to flow properly into the bond line and provide sufficient and homogeneous coverage of the bonding surface area, but it will often also affect the ability of the adhesive to form a strong and reliable bond. The most common unit of measuring the surface energy of a material is the dyne. Using dyne pens, this value can be found for virtually any material. Certain materials, especially common injection-moulded plastics such as PE, will have low surface energies.
13) Joe Mausar, ‘On the Surface - Surface Energy and Surface Tension,’ Adhesives and Sealants Industry, 2010.

